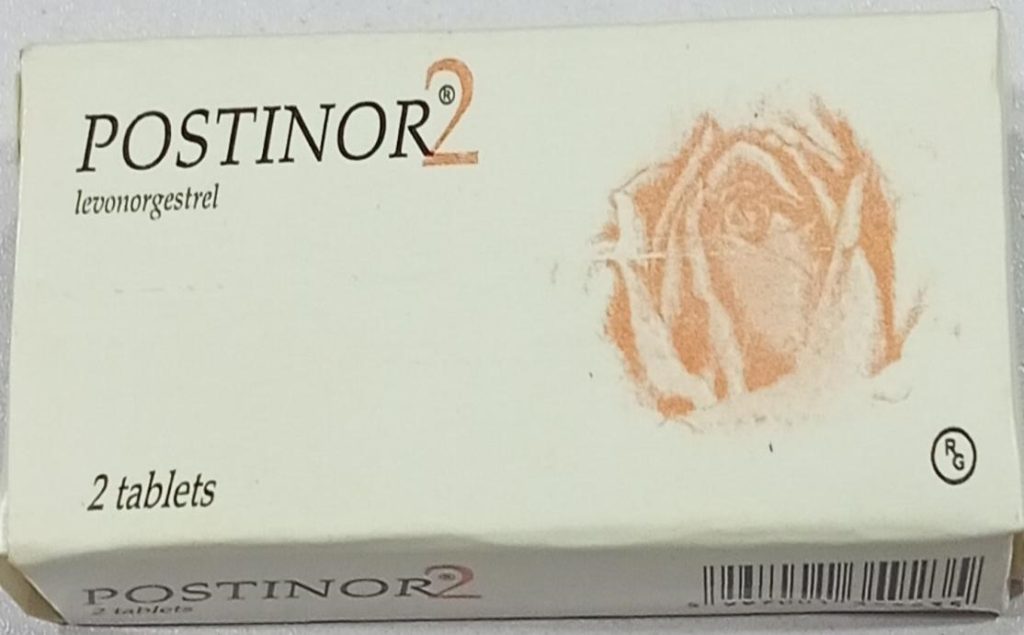
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public warning about counterfeit versions of the emergency contraceptive pill, Postinor-2 (Levonorgestrel 0.75mg), circulating in the market.
The alert made known on the agency’s website on Tuesday, August 26, follows a report from the product’s marketing authorisation holder, the Society of Family Health (SFH), which confirmed that the discovered batches are not authentic.
NAFDAC identified two types of falsified products and provided clear guidance on how to differentiate them from the genuine ones.
The primary identifiers for the fake versions are spelling errors on the packaging.
The pin verification sticker on the counterfeit product has a smaller font and misspells “Verify” as “Veify.”
Additionally, the back of the pack on the fake product misspells “Distributed” as “Distnibuted.” The original product has a larger, more visible font and correct spellings.
According to the agency, the genuine product is identified by batch number T32458H, with a manufacturing date of February 2023 and an expiry date of February 2027.
NAFDAC stated that the falsified products were released in different versions with varying details.
The first counterfeit type carried Batch Number T36184B, with a manufacturing date of August 2024 and an expiry date of August 2028.
The second counterfeit type carried Batch Number 332, manufactured in March 2023 and set to expire in February 2027.
NAFDAC warns that the use of these falsified products poses significant health and safety risks.
The agency noted that they may contain incorrect, substandard, or toxic ingredients and may not have been manufactured under sterile conditions.
According to NAFDAC, potential risks include failure of the contraceptive to work, harmful contaminants leading to allergic reactions, organ damage, or death, long-term reproductive health complications and missed opportunity for genuine emergency contraception.

In response to the discovery, NAFDAC has directed all its zonal and state coordinators to conduct surveillance and mop up the falsified products from circulation.
Also Read:
The agency said investigations into the source of the counterfeit goods are ongoing.
The agency urged distributors, retailers, and healthcare professionals to be vigilant and verify the authenticity of all medical products they handle.
Consumers are also advised to only purchase medicines from authorised and licensed suppliers and to carefully check the product’s physical condition and authenticity.
NAFDAC encouraged the public and healthcare providers to report any suspicion of the sale of substandard medicines or any related adverse side effects.
“Reports can be made to the nearest NAFDAC office, through the toll-free number 0800-162-3322, or via email at [email protected]. Adverse events can also be reported through NAFDAC’s e-reporting platforms on its website or the Med-safety application.
“This alert will also be submitted to the World Health Organisation (WHO) Global Surveillance and Monitoring System (GSMS) to notify the international community”, said NAFDAC.




